
Chemistry, 15.01.2021 14:00 jacobbrandon2002
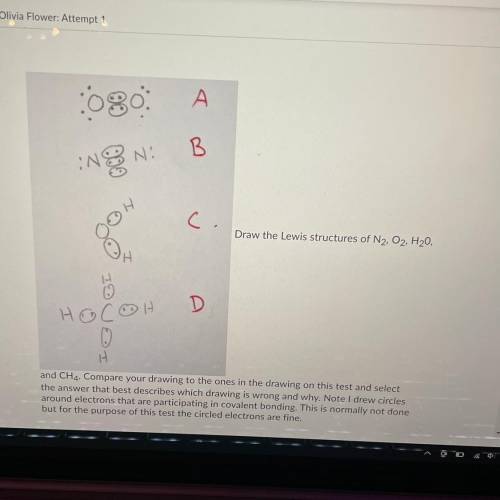
Draw the Lewis structures of N2, O2, H20, and CH4
Compare your drawing to the ones in the drawing on this test and select the answer that best describes which drawing is wrong and why.
A: O2 is wrong because it shows the electrons at a 45 degree angle to the Oxygen atoms
B: N2 is wrong because it shows a triple bond
C: H2O is wrong because it is missing 4 valence electrons
D: CH4 is wrong because the bonds are supposed to be bent at 109.5 degrees

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2


Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Draw the Lewis structures of N2, O2, H20, and CH4
Compare your drawing to the ones in the drawing o...
Questions










Computers and Technology, 03.08.2019 04:20












