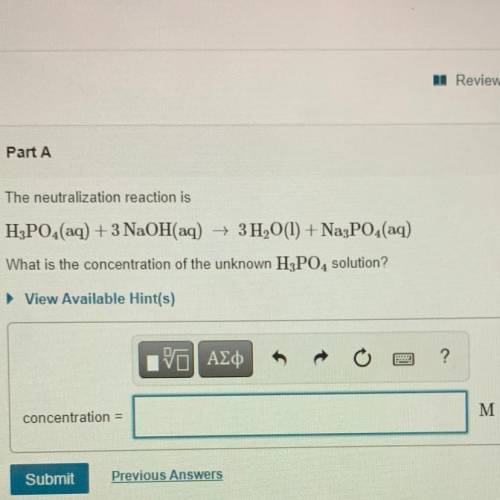
The neutralization reaction is
H3PO4(aq) + 3 NaOH(aq) + 3H2O(l) + Na3PO4(aq)
What is the conc...

Chemistry, 15.01.2021 07:00 SauceyNaee
The neutralization reaction is
H3PO4(aq) + 3 NaOH(aq) + 3H2O(l) + Na3PO4(aq)
What is the concentration of the unknown H3PO4 solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

You know the right answer?
Questions



Mathematics, 22.03.2021 01:30



Social Studies, 22.03.2021 01:30

Mathematics, 22.03.2021 01:30

Mathematics, 22.03.2021 01:30


Mathematics, 22.03.2021 01:40


Mathematics, 22.03.2021 01:40

Mathematics, 22.03.2021 01:40

French, 22.03.2021 01:40

Mathematics, 22.03.2021 01:40

Arts, 22.03.2021 01:40

Mathematics, 22.03.2021 01:40

History, 22.03.2021 01:40

Mathematics, 22.03.2021 01:40

Mathematics, 22.03.2021 01:40



