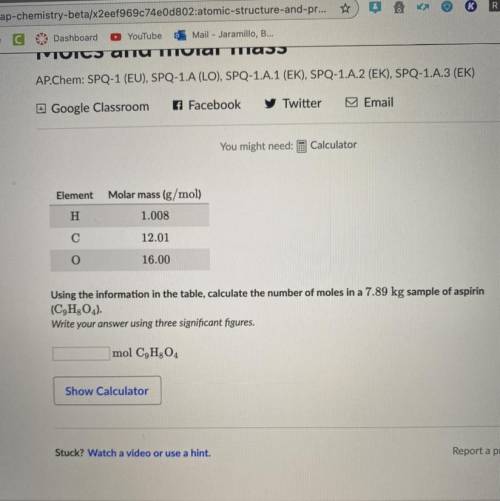
Element
Molar mass (g/mol)
H
1.008
С
12.01
O
16.00
Using...

Chemistry, 14.01.2021 19:10 FailingstudentXD
Element
Molar mass (g/mol)
H
1.008
С
12.01
O
16.00
Using the information in the table, calculate the number of moles in a 7.89 kg sample of aspirin (C9H2O4).
Write your answer using three significant figures.
mol C9H304
Show Calculator

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2


You know the right answer?
Questions

History, 28.07.2019 16:00


Health, 28.07.2019 16:00

Arts, 28.07.2019 16:00

English, 28.07.2019 16:00

Mathematics, 28.07.2019 16:00


History, 28.07.2019 16:00



Biology, 28.07.2019 16:00


Chemistry, 28.07.2019 16:00




Biology, 28.07.2019 16:00





