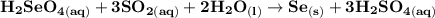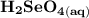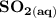Chemistry, 14.01.2021 16:40 angelreji386
Write a balanced equation describing the reduction of H2SeO4 by SO2 to produce selenium. (Use the lowest possible coefficients. Include states-of-matter under SATP conditions in your answer.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3


Chemistry, 23.06.2019 13:30
How does water evaporating from a glass show that matter is made up of particles? a. the heat energy from the air causes the glass to fill up with water particles. b. the liquid water particles turn into water vapor that spreads in the air. c. the particles of the glass dissolve in water and cause it to evaporate. d. the tiny particles of the glass evaporate and seem to disappear.
Answers: 2
You know the right answer?
Write a balanced equation describing the reduction of H2SeO4 by SO2 to produce selenium. (Use the lo...
Questions

Spanish, 03.03.2021 21:40

Biology, 03.03.2021 21:40

Mathematics, 03.03.2021 21:40


Mathematics, 03.03.2021 21:40


Geography, 03.03.2021 21:40

Mathematics, 03.03.2021 21:40


Spanish, 03.03.2021 21:40

Mathematics, 03.03.2021 21:40

SAT, 03.03.2021 21:40

Spanish, 03.03.2021 21:40

Mathematics, 03.03.2021 21:40

Biology, 03.03.2021 21:40

Mathematics, 03.03.2021 21:40

Chemistry, 03.03.2021 21:40



Social Studies, 03.03.2021 21:40