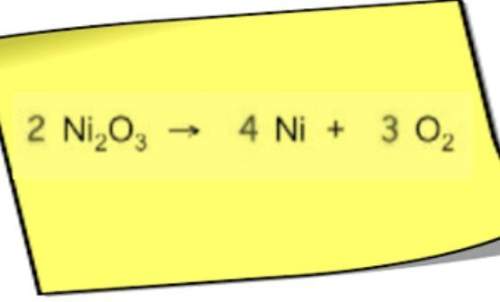
Chemistry, 14.01.2021 14:00 edenszigethyow8ajk
The elementary liquid-phase reversible reaction is carried out
isothermically. A↔3C. Pure A enters at a temperature of 400 K and a pressure of
10 atm. At this temperature, KC = 0.25(mol/dm3)2. Write the rate law and then
calculate the equilibrium conversion for each of the following situations
(a) The reaction is carried out in a constant-volume batch reactor.
(b) The reaction is carried out in a constant-pressure batch reactor.
(c) Can you explain the reason why there would be a difference in the two values of the equilibrium conversion?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 05:30
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table? atom p has an estimated zeff of 7 and is therefore to the left of atom q, which has a zeff of 6. atom p has an estimated zeff of 7 and is therefore to the right of atom q, which has a zeff of 6. atom p has an estimated zeff of 5 and is therefore below atom q, which has a zeff of 4. atom p has an estimated zeff of 5 and is therefore above atom q, which has a zeff of 4.
Answers: 3
You know the right answer?
The elementary liquid-phase reversible reaction is carried out
isothermically. A↔3C. Pure A enters...
Questions

Mathematics, 13.01.2021 02:10

Biology, 13.01.2021 02:10

Arts, 13.01.2021 02:10


Mathematics, 13.01.2021 02:10


Mathematics, 13.01.2021 02:10

Mathematics, 13.01.2021 02:10





Chemistry, 13.01.2021 02:10


Chemistry, 13.01.2021 02:10

Mathematics, 13.01.2021 02:10

Mathematics, 13.01.2021 02:10