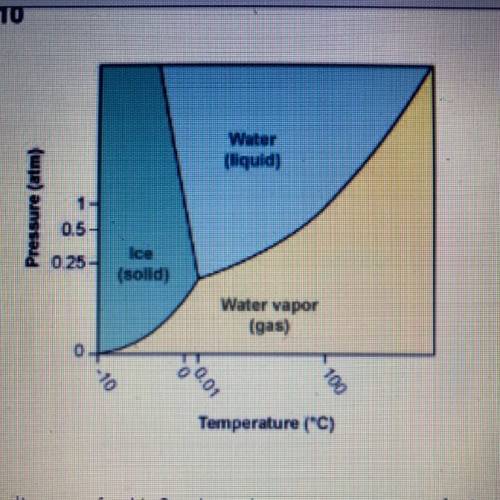
Using the phase diagram for H20, what phase is water in at 1 atm pressure
and 150°C?
A....


Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
Questions


Health, 28.01.2021 20:20


Chemistry, 28.01.2021 20:20

History, 28.01.2021 20:20


Computers and Technology, 28.01.2021 20:20

English, 28.01.2021 20:20




Mathematics, 28.01.2021 20:20

Chemistry, 28.01.2021 20:20

Health, 28.01.2021 20:20


History, 28.01.2021 20:20

Mathematics, 28.01.2021 20:20

Mathematics, 28.01.2021 20:20

Physics, 28.01.2021 20:20

English, 28.01.2021 20:20