
Chemistry, 14.01.2021 01:00 mocheal8216
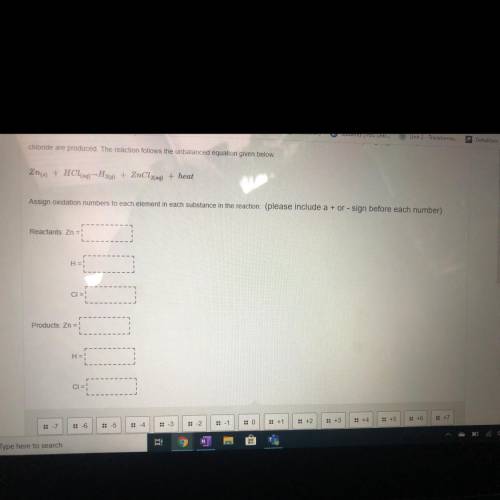
Chloride are produced. The reaction follows the unbalanced equation given below.
Zn(s) + HCl(a) --H26) + ZnCl2(aq) + heat
Assign oxidation numbers to each element in each substance in the reaction (please include a + or - sign before each number)
Reactants: Zn = !
H =
CIE:
Products: Zn =
H = !
CI=
:: +3
: 0
.: +5
:: -7
:: -5
:: -2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1
You know the right answer?
Chloride are produced. The reaction follows the unbalanced equation given below.
Zn(s) + HCl(a) --H...
Questions


Mathematics, 14.01.2020 03:31

Chemistry, 14.01.2020 03:31



Biology, 14.01.2020 03:31

Mathematics, 14.01.2020 03:31

English, 14.01.2020 03:31

Mathematics, 14.01.2020 03:31

Mathematics, 14.01.2020 03:31

Mathematics, 14.01.2020 03:31


Mathematics, 14.01.2020 03:31







Mathematics, 14.01.2020 03:31



