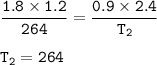Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2


Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
A gas has 1.2 L of volume, 1.8 atm of pressure and 264 K temperature. If both the
volume was double...
Questions

Mathematics, 29.09.2020 14:01


English, 29.09.2020 14:01


Mathematics, 29.09.2020 14:01

History, 29.09.2020 14:01



Social Studies, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01


History, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Arts, 29.09.2020 14:01

History, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01