
Chemistry, 13.01.2021 19:00 einstein101
HELP ASAP
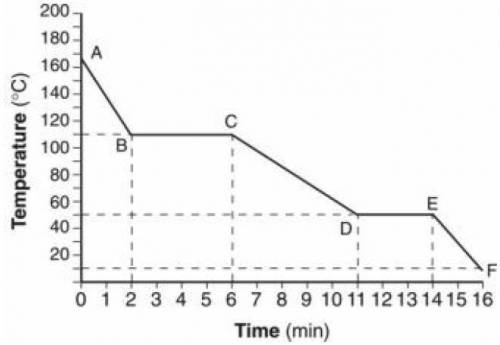
The graph represents the relationship between temperature and time as energy is removed from a sample of H2O.
Which statement correctly describes the energy of the particles of the sample during interval D–E?
Potential energy decreases and average kinetic energy increases.
Potential energy decreases and average kinetic energy increases.
Potential energy increases and average kinetic energy increases.
Potential energy increases and average kinetic energy increases.
Potential energy decreases and average kinetic energy remains the same.
Potential energy decreases and average kinetic energy remains the same.
Potential energy remains the same and average kinetic energy increases.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
HELP ASAP
The graph represents the relationship between temperature and time as energy is removed f...
Questions

Biology, 18.10.2020 06:01



Mathematics, 18.10.2020 06:01







Chemistry, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01




Mathematics, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01

Biology, 18.10.2020 06:01



