Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
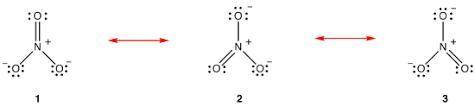
Draw the Lewis structures for three resonance forms of the nitrate ion, NO−3 . Include electron lone...
Questions


Mathematics, 18.05.2020 11:57

Chemistry, 18.05.2020 11:57

History, 18.05.2020 11:57

Mathematics, 18.05.2020 11:57



English, 18.05.2020 11:57

Mathematics, 18.05.2020 11:57


Social Studies, 18.05.2020 11:57

Computers and Technology, 18.05.2020 11:57

Mathematics, 18.05.2020 11:57


Mathematics, 18.05.2020 11:57

Mathematics, 18.05.2020 11:57



English, 18.05.2020 11:57

Mathematics, 18.05.2020 11:57