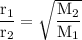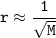The molar masses of four unknown gases are shown in the table.
Molar Mass of Unknown Gases
G...

Chemistry, 13.01.2021 17:10 alexmiranda00
The molar masses of four unknown gases are shown in the table.
Molar Mass of Unknown Gases
Gas Molar Mass
A 44 g/mol
B 20 g/mol
C 30 g/mol
D 32 g/mol
Which gas is likely to have the lowest rate of diffusion?
a. Gas A
b. Gas B
c. Gas C
d. Gas D

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Questions












Mathematics, 15.07.2020 14:01


Mathematics, 15.07.2020 14:01

Mathematics, 15.07.2020 14:01


Physics, 15.07.2020 14:01


Mathematics, 15.07.2020 14:01