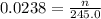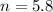Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

You know the right answer?
A chemist adds of a aluminum chloride solution to a reaction flask. Calculate the millimoles of alum...
Questions

Mathematics, 05.05.2021 01:00


Mathematics, 05.05.2021 01:00



Social Studies, 05.05.2021 01:00

Social Studies, 05.05.2021 01:00



Chemistry, 05.05.2021 01:00





Mathematics, 05.05.2021 01:00


Mathematics, 05.05.2021 01:00



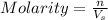
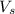 = volume of solution in ml
= volume of solution in ml