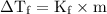Chemistry, 13.01.2021 07:20 thelordoftheknowwjo4
Which of the following aqueous solutions would have the greatest freezing-point depression?
(A) 0.10 m (NH₄)₂SO₄
(B) 0.10 m MnSO₄
(C) 0.10 m NaF
(D) 0.10 m KCI
(E) 0.10 m CH₃OH

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 23.06.2019 09:00
Agust of wind blowing east pushes against a ball. when will the wind do work on the ball? when the ball moves to the east when the ball moves to the north when the ball stays in one place when the ball moves north or south
Answers: 1
You know the right answer?
Which of the following aqueous solutions would have the greatest freezing-point depression?
(A) 0.1...
Questions

Health, 08.12.2020 02:30

Mathematics, 08.12.2020 02:30


English, 08.12.2020 02:30


Computers and Technology, 08.12.2020 02:30




Computers and Technology, 08.12.2020 02:30



Mathematics, 08.12.2020 02:30

Business, 08.12.2020 02:30

Biology, 08.12.2020 02:30



Mathematics, 08.12.2020 02:30


Mathematics, 08.12.2020 02:30