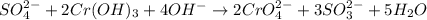Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
SO42- + 2Cr(OH)3 + 4OH- → + 2CrO42- + 3SO32- + 5H2O
In the above redox reaction. Use oxidation nu...
Questions

Chemistry, 18.04.2021 19:30



Mathematics, 18.04.2021 19:30

Mathematics, 18.04.2021 19:30



Mathematics, 18.04.2021 19:30

History, 18.04.2021 19:30

Mathematics, 18.04.2021 19:30

English, 18.04.2021 19:30

History, 18.04.2021 19:30

Mathematics, 18.04.2021 19:30



History, 18.04.2021 19:30

Biology, 18.04.2021 19:30



Mathematics, 18.04.2021 19:30