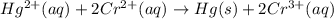Chemistry, 12.01.2021 17:20 baneenbilal8510
Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron-transfer reaction. Hg^2+(aq) + 2Cr^2+(aq) → Hg(s) + 2Cr^3+(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in th...
Questions

Spanish, 26.06.2019 05:30

English, 26.06.2019 05:30



English, 26.06.2019 05:30



Mathematics, 26.06.2019 05:30

Mathematics, 26.06.2019 05:30


History, 26.06.2019 05:30

English, 26.06.2019 05:30

Computers and Technology, 26.06.2019 05:30

History, 26.06.2019 05:30




Health, 26.06.2019 05:30

English, 26.06.2019 05:30