Do this Q7 if someone do I will her or him brainliest
...

Chemistry, 12.01.2021 17:00 kayleewoodard
Do this Q7 if someone do I will her or him brainliest
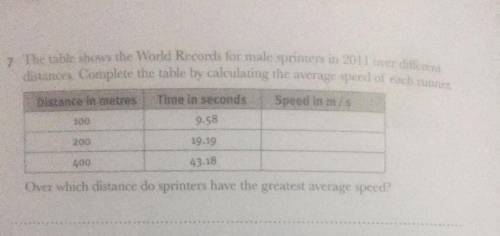

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
Questions



English, 30.10.2020 05:50




Chemistry, 30.10.2020 05:50


Mathematics, 30.10.2020 05:50


Computers and Technology, 30.10.2020 05:50




Mathematics, 30.10.2020 05:50

Biology, 30.10.2020 05:50


Mathematics, 30.10.2020 05:50


English, 30.10.2020 05:50



