
Chemistry, 15.01.2020 15:31 aidanfbussiness
1. in the reaction c5h8 + o2 --> 8h2o + 5co2, what coefficient should be placed in front of o2 to balance the reaction?
a. 5
b. 8
c. 9
d. no coefficient is needed
2. how does the law of conservation of mass apply to this reaction: c2h4 + o2 --> 2h2o + 2co2?
a. each element needs to be balanced.
b. the equation needs to be balanced. there are fewer oxygen atons in the equation than hydrogen or carbon.
c. only the oxygen needs to be balanced. there are equal numbers of hydrogen and carbon.
d. the law of conservation of mass has already been applied. there is an equal number of each element on both sides of the equation
3. how does the law of conservation of mass apply to this reaction: mg + hcl --> h2 + mgcl2?
a. the law of conservation of mass has already been applied. there is an equal number of each element on both sides of the equation.
b. only the hydrogen needs to be balanced. there are equal amount of magnesium and chlorine.
c. hydrogen and chlorine need to be balanced. there is an equal amount of magnesium on each side.
d. the equation needs to be balanced. there are a few hydrogen atoms in the equation than magnesium or chlorine.
4. which of these is an example of a solution?
a. oil and vinegar in a salad dressing jar
b. a glass of water with ice in it
c. sugar stirred in tea
d. sand stirred in water

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
1. in the reaction c5h8 + o2 --> 8h2o + 5co2, what coefficient should be placed in front of o2...
Questions

History, 12.07.2019 12:30

Mathematics, 12.07.2019 12:30


Health, 12.07.2019 12:30

History, 12.07.2019 12:30

Mathematics, 12.07.2019 12:30



Mathematics, 12.07.2019 12:30





History, 12.07.2019 12:30



Biology, 12.07.2019 12:30


Business, 12.07.2019 12:30

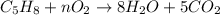
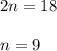
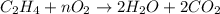
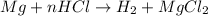
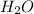 So, this is not considered as a solution.
So, this is not considered as a solution.


