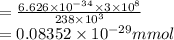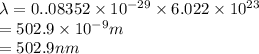Chemistry, 11.01.2021 15:40 vanessacox45
It takes to break a carbon-iodine single bond. Calculate the maximum wavelength of light for which a carbon-iodine single bond could be broken by absorbing a single photon.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
It takes to break a carbon-iodine single bond. Calculate the maximum wavelength of light for which a...
Questions

Chemistry, 09.10.2021 14:00

History, 09.10.2021 14:00




SAT, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00



Mathematics, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00


History, 09.10.2021 14:00

Chemistry, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00


History, 09.10.2021 14:00

Chemistry, 09.10.2021 14:00

History, 09.10.2021 14:00