
Chemistry, 11.01.2021 04:10 makennahudson94
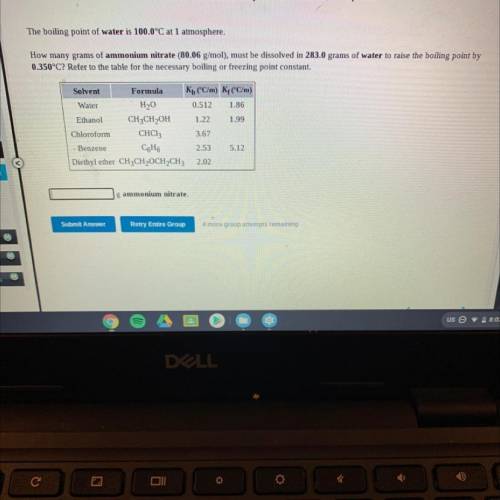
The boiling point of water is 100.0°C at 1 atmosphere.
How many grams of ammonium nitrate (80.06 g/mol), must be dissolved in 283.0 grams of water to raise the boiling point by
0.350°C? Refer to the table for the necessary boiling or freezing point constant.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
The boiling point of water is 100.0°C at 1 atmosphere.
How many grams of ammonium nitrate (80.06 g/...
Questions



English, 13.01.2021 17:10

History, 13.01.2021 17:10



Mathematics, 13.01.2021 17:10

Geography, 13.01.2021 17:10





Mathematics, 13.01.2021 17:10









