
Chemistry, 10.01.2021 22:20 Itsyourgirllulu
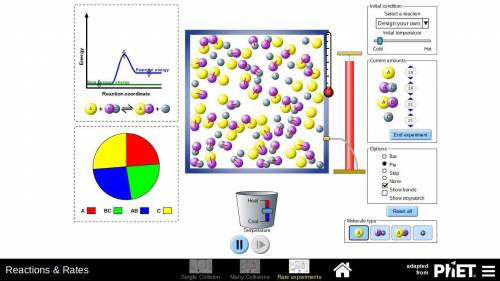
In part D of task 1, you identified at least two ways in which the reaction of nitrogen and hydrogen could be changed to alter the equilibrium. Use the simulation to test those changes. Describe how you used the simulation to model the changes and the results they produced. Use these methods if you find them helpful:
Look at the pie graph to see how the system changes.
Use the Temperature slider at the bottom to cool or heat the mixture.
Click the pause button on the simulation to observe the number of particles at any point of time.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3

Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3
You know the right answer?
In part D of task 1, you identified at least two ways in which the reaction of nitrogen and hydrogen...
Questions

English, 31.01.2020 15:49

Social Studies, 31.01.2020 15:50

Mathematics, 31.01.2020 15:50


World Languages, 31.01.2020 15:50



Mathematics, 31.01.2020 15:50

English, 31.01.2020 15:50






Mathematics, 31.01.2020 15:50

Mathematics, 31.01.2020 15:50

Mathematics, 31.01.2020 15:50



Social Studies, 31.01.2020 15:50



