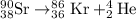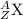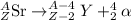Chemistry, 10.01.2021 08:50 kenziepickup
9. Write a balanced nuclear equation for the following: The isotope Strontium-90 decays by Q-
decay (1 point)
OfSr — He +56 kr
OS → H +39 kr
o Sr + Be +38kr
99 Sr → He +3 Se
SAST +7+99 Sr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
9. Write a balanced nuclear equation for the following: The isotope Strontium-90 decays by Q-
decay...
Questions

Computers and Technology, 28.07.2019 00:30



Social Studies, 28.07.2019 00:30

Biology, 28.07.2019 00:30

Mathematics, 28.07.2019 00:30

Biology, 28.07.2019 00:30

Mathematics, 28.07.2019 00:30


Health, 28.07.2019 00:30





History, 28.07.2019 00:30




Computers and Technology, 28.07.2019 00:30

Social Studies, 28.07.2019 00:30