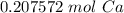How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are provided below. It is your responsibility to know which conversion factor to use!
Avogadro’s number: 6.02x1023 atoms = 1 mole
Molar mass of calcium: 40.078 g Ca = 1 mol Ca
A. 3.12x10^21 mol
B. 0.21 mol
C. 3.12 mol
D. 0.21x10^21 mol

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are...
Questions

English, 12.11.2020 14:20


Mathematics, 12.11.2020 14:20




Computers and Technology, 12.11.2020 14:20

Mathematics, 12.11.2020 14:20

Mathematics, 12.11.2020 14:20


English, 12.11.2020 14:20



Chemistry, 12.11.2020 14:30




History, 12.11.2020 14:30

Social Studies, 12.11.2020 14:30

Mathematics, 12.11.2020 14:30

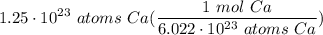 Multiply:
Multiply: