
Chemistry, 09.01.2021 23:30 Naysa150724
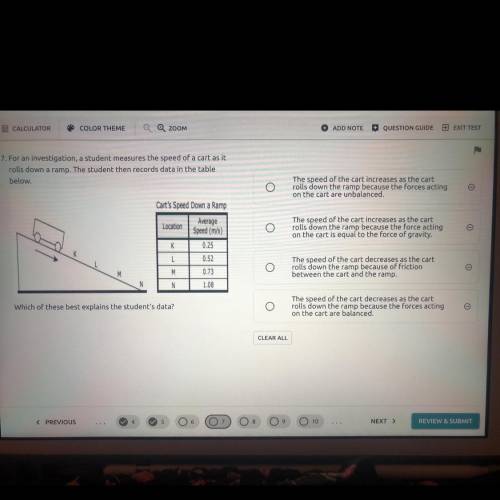
7. For an investigation, a student measures the speed of a cart as it
rolls down a ramp. The student then records data in the table
below.
Cart's Speed Down a Ramo
Average
Location
Speed (mis)
0.25
0.73
1.08
Which of these best explains the student's data?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
7. For an investigation, a student measures the speed of a cart as it
rolls down a ramp. The studen...
Questions



Mathematics, 03.11.2020 01:10








Mathematics, 03.11.2020 01:10

Mathematics, 03.11.2020 01:10

Chemistry, 03.11.2020 01:10


English, 03.11.2020 01:10

Mathematics, 03.11.2020 01:10

Mathematics, 03.11.2020 01:10

Mathematics, 03.11.2020 01:10

English, 03.11.2020 01:10

Computers and Technology, 03.11.2020 01:10



