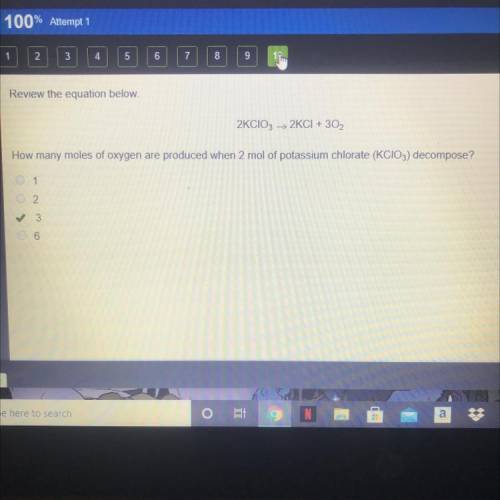
Review the equation below.
2KCIO3 > 2KCI + 3O2
How many moles of oxygen are produced...

Chemistry, 09.01.2021 22:10 tessalopezgarcia2345
Review the equation below.
2KCIO3 > 2KCI + 3O2
How many moles of oxygen are produced when 2 mol of potassium chlorate (KCIO3) decompose?
A. 1
B. 2
C. 3
D. 6
The correct answer is C on edge 2021

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Questions

English, 06.10.2019 23:30

History, 06.10.2019 23:30

Physics, 06.10.2019 23:30

Computers and Technology, 06.10.2019 23:30


Mathematics, 06.10.2019 23:30

Mathematics, 06.10.2019 23:30


History, 06.10.2019 23:30

Mathematics, 06.10.2019 23:30


History, 06.10.2019 23:30

Mathematics, 06.10.2019 23:30

Mathematics, 06.10.2019 23:30



Mathematics, 06.10.2019 23:30

SAT, 06.10.2019 23:30

Mathematics, 06.10.2019 23:30



