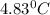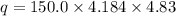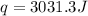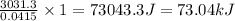2
3 of 6 - SCH4U
Date: Name(s):
4. HCl is a corrosive colourless gas that dissolves rea...

Chemistry, 09.01.2021 06:20 saleenhernandez83
2
3 of 6 - SCH4U
Date: Name(s):
4. HCl is a corrosive colourless gas that dissolves readily in water.
Aqueous HCl reacts with NaOH to form water and NaCl. In a simple
calorimeter, a 100.00 mL sample of 0.415 mol/L HCl(aq) is mixed
with 50.00 mL of excess NaOH(aq). During the reaction, there is a
rise in temperature by 4.83 °C. Calculate the molar enthalpy change
(in kJ/mol) for the above reaction. SHOW ALL YOUR WORK.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

You know the right answer?
Questions

English, 03.02.2021 01:00

Health, 03.02.2021 01:00

Spanish, 03.02.2021 01:00



Chemistry, 03.02.2021 01:00

Chemistry, 03.02.2021 01:00










Social Studies, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00


English, 03.02.2021 01:00


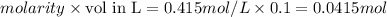
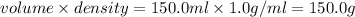
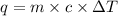
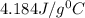
 = change in temperature =
= change in temperature =