
Chemistry, 09.01.2021 02:20 serenityjohnson98765
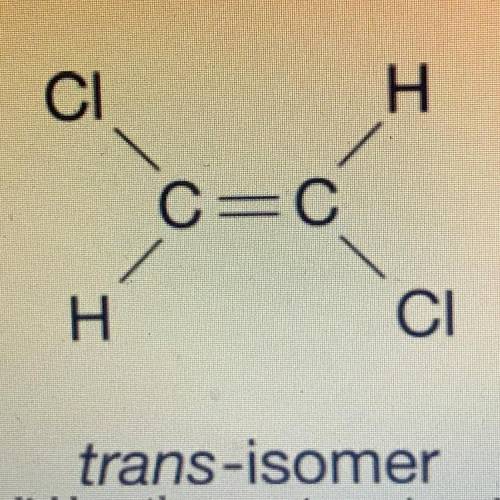
The structural formula for two isomers of 1, 2-dichloroethene are shown above. which of the two liquids has the higher equilibrium vapor pressure at 20° C and why
a) the cis-omer because it only has dipole dipole and the trans-iomer has london dispersion forces
b) cis-omer because it has only ldf and trans-iomer has dipole dipole
c) trans-iomer because it has only dipole dipole and cis-omer has ldf
d) trans-iomer because it has only ldf and cis-omer has dipole dipole

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
You know the right answer?
The structural formula for two isomers of 1, 2-dichloroethene are shown above. which of the two liqu...
Questions


Chemistry, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50


Mathematics, 17.11.2020 19:50


Mathematics, 17.11.2020 19:50


Health, 17.11.2020 19:50

Physics, 17.11.2020 19:50

Computers and Technology, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50




Mathematics, 17.11.2020 19:50

Chemistry, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50



