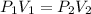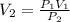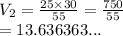Chemistry, 08.01.2021 18:00 cupcake3103670
-) A sample of oxygen had an initial volume of 25.0L and was at a standard pressure of 30atm, what would the new volume be if the pressure was increased to 55.00 atm?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
-) A sample of oxygen had an initial volume of 25.0L and was at a standard pressure of
30atm, what...
Questions

Mathematics, 10.02.2021 21:40

Mathematics, 10.02.2021 21:40

History, 10.02.2021 21:40

Mathematics, 10.02.2021 21:40

History, 10.02.2021 21:40

History, 10.02.2021 21:40

Mathematics, 10.02.2021 21:40


Arts, 10.02.2021 21:40


Computers and Technology, 10.02.2021 21:40

Arts, 10.02.2021 21:40

Biology, 10.02.2021 21:40

Mathematics, 10.02.2021 21:40

Mathematics, 10.02.2021 21:40




Physics, 10.02.2021 21:40

Mathematics, 10.02.2021 21:40