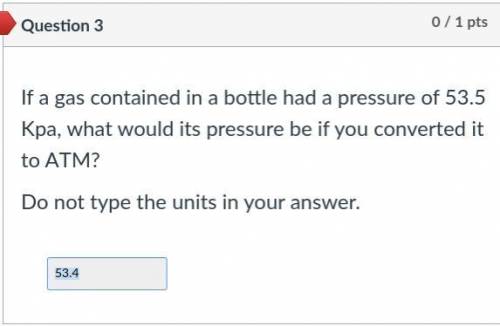
The answer is not 53.4. Please help.
...

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Questions


History, 02.04.2021 01:00

Law, 02.04.2021 01:00


Social Studies, 02.04.2021 01:00

Mathematics, 02.04.2021 01:00

Law, 02.04.2021 01:00


English, 02.04.2021 01:00

Chemistry, 02.04.2021 01:00



Mathematics, 02.04.2021 01:00




Mathematics, 02.04.2021 01:00

Biology, 02.04.2021 01:00


Advanced Placement (AP), 02.04.2021 01:00