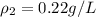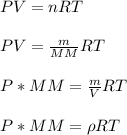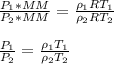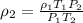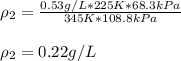Chemistry, 08.01.2021 05:10 mendezmarco2004
A sample of gas has a density of 0.53 g/L at 225 K and under a pressure of 108.8 kPa. Find the density of the gas at 345 K under a pressure of 68.3 kPa. Assuming the mass is equal to 1 gram.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
A sample of gas has a density of 0.53 g/L at 225 K and under a pressure of 108.8 kPa. Find the densi...
Questions





Mathematics, 11.01.2021 22:40

Mathematics, 11.01.2021 22:40

Engineering, 11.01.2021 22:40

English, 11.01.2021 22:40

Mathematics, 11.01.2021 22:40

Geography, 11.01.2021 22:40


Physics, 11.01.2021 22:40

Mathematics, 11.01.2021 22:40

Biology, 11.01.2021 22:40

Social Studies, 11.01.2021 22:40


Mathematics, 11.01.2021 22:40

Mathematics, 11.01.2021 22:40

Mathematics, 11.01.2021 22:40

Mathematics, 11.01.2021 22:40