
Chemistry, 08.01.2021 02:40 elondamason
Suppose a lab procedure calls for 0.75 L of a 0.25 M CaCl2 solution. How much of a 10.0 M stock solution do we dilute? Where the diluted solution is #2 (the numerator)
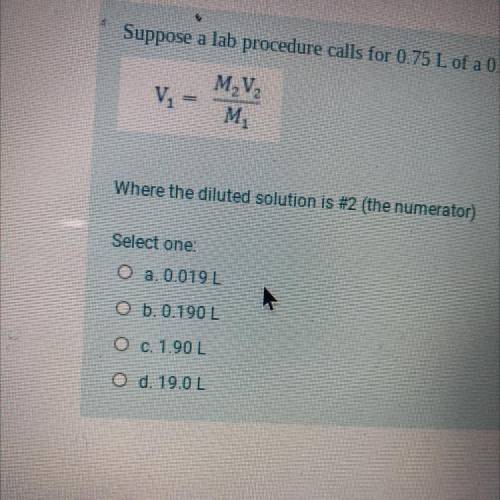

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
You know the right answer?
Suppose a lab procedure calls for 0.75 L of a 0.25 M CaCl2 solution. How much of a 10.0 M stock solu...
Questions

Mathematics, 28.10.2020 19:20

English, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20






History, 28.10.2020 19:20




Biology, 28.10.2020 19:20

Health, 28.10.2020 19:20


Mathematics, 28.10.2020 19:20


Social Studies, 28.10.2020 19:20


Mathematics, 28.10.2020 19:20



