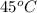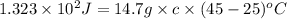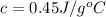An unknown substance has a mass of 14.7 g . when the substance absorbs 1.323×102 j of heat, the temperature of the substance is raised from 25.0 ∘c to 45.0 ∘c . what is the most likely identity of the substance?
a. aluminum 0.903
b. iron 0.449
c. water 4.184
d. silver 0.235
e. copper 0.385
f. ethanol 2.42

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
An unknown substance has a mass of 14.7 g . when the substance absorbs 1.323×102 j of heat, the temp...
Questions

Mathematics, 15.01.2021 18:00

English, 15.01.2021 18:00




Mathematics, 15.01.2021 18:00


Mathematics, 15.01.2021 18:00

Mathematics, 15.01.2021 18:00



Mathematics, 15.01.2021 18:00

Mathematics, 15.01.2021 18:00



Mathematics, 15.01.2021 18:00


Advanced Placement (AP), 15.01.2021 18:00


Social Studies, 15.01.2021 18:00

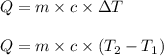
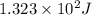
 = change in temperature
= change in temperature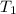 = initial temperature =
= initial temperature = 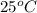
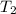 = final temperature =
= final temperature =