
Chemistry, 07.01.2021 21:30 michellealvarez985
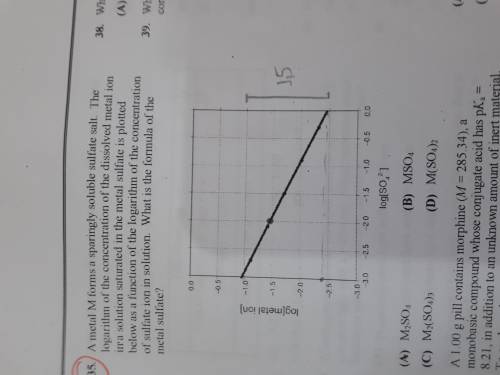
A metal M forms a sparingly soluble sulfate salt. The logarithm of the concentration of the dissolved metal ion in a solution saturated in the metal sulfate is plotted bellow as a function of the logarithm of the concentration of sulfate ion in solution. What is the formula of the metal sulfate?
a)M2SO4
b)MSO4
c)M2(SO4)3
d) M(SO4)2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

You know the right answer?
A metal M forms a sparingly soluble sulfate salt. The logarithm of the concentration of the dissolve...
Questions

Mathematics, 21.04.2020 21:47




English, 21.04.2020 21:47

Chemistry, 21.04.2020 21:47

Mathematics, 21.04.2020 21:47




Computers and Technology, 21.04.2020 21:47


Mathematics, 21.04.2020 21:47



Mathematics, 21.04.2020 21:47

Chemistry, 21.04.2020 21:47



English, 21.04.2020 21:47



