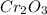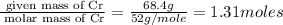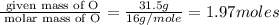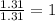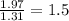Chemistry, 07.01.2021 17:30 elizabethatkins1922
Measurements show that unknown compound X has the following composition: element mass % chromium 68.4% oxygen 31.5% Write the empirical chemical formula of X.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1


Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Measurements show that unknown compound X has the following composition: element mass % chromium 68....
Questions

English, 01.07.2019 21:30

Mathematics, 01.07.2019 21:30

History, 01.07.2019 21:30

Mathematics, 01.07.2019 21:30

Social Studies, 01.07.2019 21:30


Computers and Technology, 01.07.2019 21:30



English, 01.07.2019 21:30

History, 01.07.2019 21:30

Mathematics, 01.07.2019 21:30



Mathematics, 01.07.2019 21:30

Biology, 01.07.2019 21:30