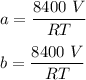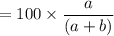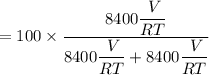Chemistry, 07.01.2021 17:30 dawsgreer4302
A mixture of ethyne gas (C2H2) and methane gas (CH4) occupied a certain volume at a total pressure of 16.8 kPa. When the sample burned, the products were CO2 gas and H2O vapor. The CO2 was collected and its pressure found to be 25.2 kPa in the same volume and at the same temperature as the original mixture. What percentage of the original mixture was methane

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
A mixture of ethyne gas (C2H2) and methane gas (CH4) occupied a certain volume at a total pressure o...
Questions

History, 08.12.2021 05:20


Mathematics, 08.12.2021 05:20

History, 08.12.2021 05:20

Chemistry, 08.12.2021 05:20


Mathematics, 08.12.2021 05:20

Biology, 08.12.2021 05:20


Mathematics, 08.12.2021 05:20

Computers and Technology, 08.12.2021 05:20

Mathematics, 08.12.2021 05:20


Social Studies, 08.12.2021 05:20


Mathematics, 08.12.2021 05:20




Mathematics, 08.12.2021 05:20

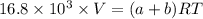
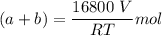 --- (1)
--- (1)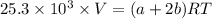
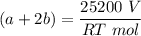 ---- (2)
---- (2)