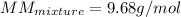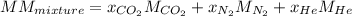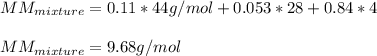Chemistry, 07.01.2021 17:00 quintasiahaskin
The gas in the discharge cell of a laser contains (in mole percent) 11% CO2, 5.3% N2, and 84% He. (a) What is the molar mass of this mixture

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
The gas in the discharge cell of a laser contains (in mole percent) 11% CO2, 5.3% N2, and 84% He. (a...
Questions

Mathematics, 10.02.2021 06:00


Chemistry, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00


History, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00



History, 10.02.2021 06:00

English, 10.02.2021 06:00