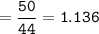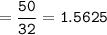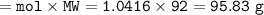Chemistry, 07.01.2021 07:20 eyeneedalife
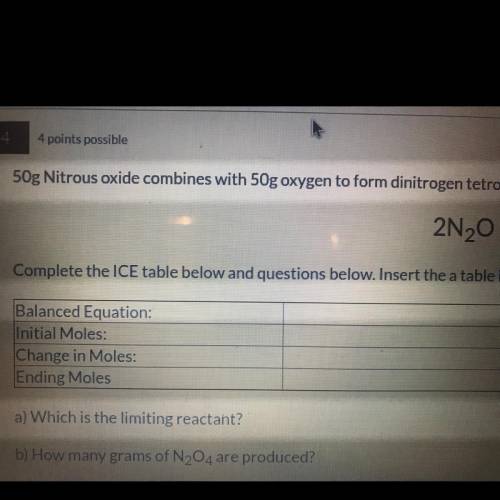
50g nitrous oxide combines with 50g oxygen form dinitrogen tetroxide according to the balanced equation below. 2N20 + 302 - 2N204

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which is true of the reactants in this displacement reaction? fe + 2hcl fecl2 + h2 a. the reactants are located to the left of the arrow in the chemical equation. b. the reactants contain 1 iron atom, 2 hydrogen atoms, and 1 chlorine atom. c. the reactants are the atoms, molecules, or compounds formed in the reaction. d. the reactants have the same physical and chemical properties as the products.
Answers: 1


Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
50g nitrous oxide combines with 50g oxygen form dinitrogen tetroxide according to the balanced equat...
Questions

Social Studies, 15.12.2020 18:40


Mathematics, 15.12.2020 18:40


History, 15.12.2020 18:40


Mathematics, 15.12.2020 18:50

Mathematics, 15.12.2020 18:50

History, 15.12.2020 18:50

Physics, 15.12.2020 18:50


Health, 15.12.2020 18:50




Mathematics, 15.12.2020 18:50

Mathematics, 15.12.2020 18:50

Mathematics, 15.12.2020 18:50


Geography, 15.12.2020 18:50