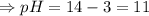Chemistry, 06.01.2021 04:40 hernan99961
2055 Q. No. 10^-2
mole of KOH is dissolved in 10 litres of
water. What will be the pH of the solution?
12
Ans: pH = 11

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

You know the right answer?
2055 Q. No. 10^-2
mole of KOH is dissolved in 10 litres of
water. What will be the pH of the s...
water. What will be the pH of the s...
Questions













Computers and Technology, 15.10.2020 08:01







Mathematics, 15.10.2020 08:01

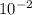
![[KOH]=\dfrac{10^{-2}}{10}\\\Rightarrow [KOH]=10^{-3}\ \text{M}](/tpl/images/1015/6356/4641b.png)
![[KOH]](/tpl/images/1015/6356/7ef8a.png) is strong base so we have the following relation
is strong base so we have the following relation![[KOH]=[OH^{-}]=10^{-3}\ \text{M}](/tpl/images/1015/6356/3b7d0.png)
![pOH=-\log [OH^{-}]=-\log10^{-3}](/tpl/images/1015/6356/43cef.png)