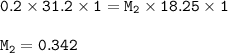Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
A 0.200 M NaOH solution was used to titrate a 18.25 mL HF
solution. The endpoint was reached after...
Questions

World Languages, 19.07.2019 04:50

History, 19.07.2019 04:50


Mathematics, 19.07.2019 04:50

English, 19.07.2019 04:50


Biology, 19.07.2019 04:50



Mathematics, 19.07.2019 04:50


History, 19.07.2019 04:50

Mathematics, 19.07.2019 04:50


History, 19.07.2019 04:50



Biology, 19.07.2019 05:00

Mathematics, 19.07.2019 05:00

History, 19.07.2019 05:00