
Chemistry, 06.01.2021 01:40 eshavaggar
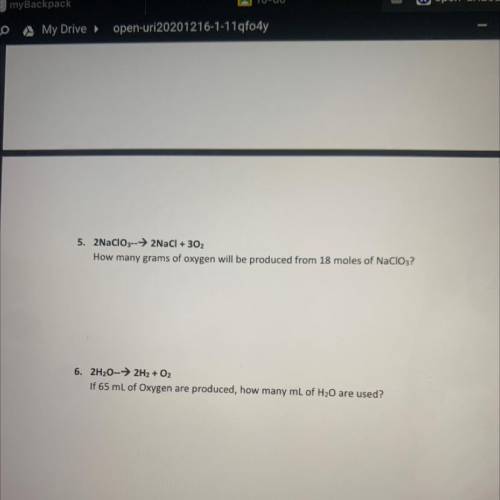
5. 2NaClO3-->2NaCl + 302
How many grams of oxygen will be produced from 18 moles of NaClO3?
6. 2H20-→ 2H2 + O2
If 65 mL of Oxygen are produced, how many mL of H20 are used?
Show all work

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
5. 2NaClO3-->2NaCl + 302
How many grams of oxygen will be produced from 18 moles of NaClO3?
Questions





English, 07.03.2020 00:23

Mathematics, 07.03.2020 00:23














Social Studies, 07.03.2020 00:23



