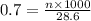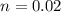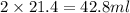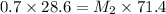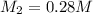Chemistry, 05.01.2021 16:50 highflylex279
You have a 0.7 M solution. Your job is to produce 50 mL of a 0.4 M solution.
A. How much of the 0.7 M solution do you need to start with? (Show your work.)
B. How many moles of solute were in the 0.7 M solution? (Show your work.)
C. How much water do you need to add to the previous amount of the 0.7 M solution to dilute it to
0.4 M? (Show your work.)
D. A student decides to put double the amount of water calculated in Part C. Describe what effect this
will have on the overall concentration of the resulting solution. Justify your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
You know the right answer?
You have a 0.7 M solution. Your job is to produce 50 mL of a 0.4 M solution.
A. How much of the 0.7...
Questions

Mathematics, 01.11.2020 17:50

Mathematics, 01.11.2020 17:50


English, 01.11.2020 17:50

English, 01.11.2020 17:50


Business, 01.11.2020 17:50


Mathematics, 01.11.2020 17:50


History, 01.11.2020 17:50

English, 01.11.2020 17:50

Biology, 01.11.2020 17:50

Computers and Technology, 01.11.2020 17:50



Computers and Technology, 01.11.2020 17:50


Mathematics, 01.11.2020 17:50

History, 01.11.2020 17:50

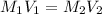
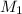 = molarity of stock solution = 0.7 M
= molarity of stock solution = 0.7 M
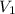 = volume of stock solution = ?
= volume of stock solution = ?
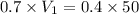
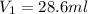
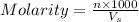
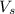 = volume of solution in ml
= volume of solution in ml