
Chemistry, 05.01.2021 16:40 Skylar4483
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
of CO2?
2C2H2(g) + 502(g) → 2H2O(g) + 4CO2(g)
A. 20.0L
B. 44.8L
C. 80.0L
D. 100 L

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1
You know the right answer?
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
Questions


Biology, 13.10.2019 16:00

Spanish, 13.10.2019 16:00

Mathematics, 13.10.2019 16:00



English, 13.10.2019 16:00


Business, 13.10.2019 16:00

Mathematics, 13.10.2019 16:00

Mathematics, 13.10.2019 16:00

Mathematics, 13.10.2019 16:00

Health, 13.10.2019 16:00

Biology, 13.10.2019 16:00






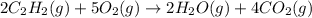
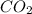 is formed by = 2 L of
is formed by = 2 L of 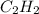
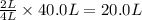 of
of 


