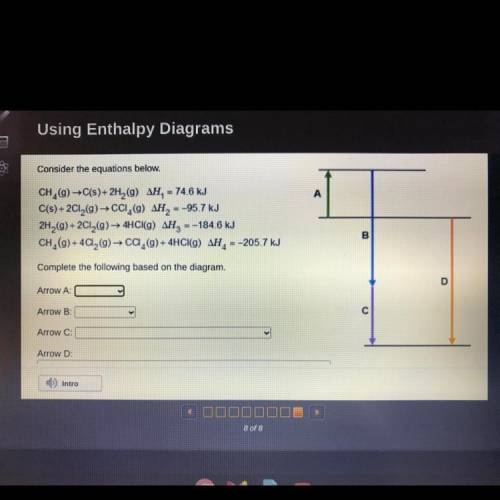
Consider the equations below.
CH, (9) →C(s)+ 2H, (9) AH, – 74.6 kJ
C(s) + 2CI,(9)→ cCi, () AH...

Chemistry, 05.01.2021 01:50 breannamiller0822
Consider the equations below.
CH, (9) →C(s)+ 2H, (9) AH, – 74.6 kJ
C(s) + 2CI,(9)→ cCi, () AH, =-95.7 kJ
2H,(g) + 2CI,(9)→ 4HCI(G) AH, =-184.6 kJ
CH, (9) + 4Ca, (9) → Ca,(9) + 4HCI(9) AH, =-205.7 kJ
Complete the following based on the diagram.
Arrow A:
Arrow B:
Arrow C:
Arrow D:

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1
You know the right answer?
Questions


Mathematics, 01.12.2020 20:30

Mathematics, 01.12.2020 20:30


Health, 01.12.2020 20:30

Mathematics, 01.12.2020 20:30






Mathematics, 01.12.2020 20:30

Social Studies, 01.12.2020 20:30






English, 01.12.2020 20:30

Health, 01.12.2020 20:30



