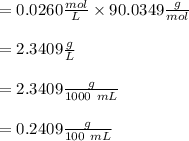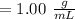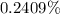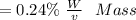Chemistry, 04.01.2021 02:00 vazquezemmy8
A sample of oxalic acid is titrated with a standardized solution of KMNO4. A 25 mL sample of oxalic acid required 12.7 mL of 0.0206 M KMnO4 to achieve a pink colored solution. The balanced equation for this reaction is shown below:
6 H+ (aq) + 2 MnO4 - (aq) + 5 H2C2O4(aq) → 10 CO2(g) +8 H2O(l) + 2Mn2+(aq)
Required:
a. What does the pink color signify in this reaction?
b. What is the ratio of MnO4 - ions to H2C2O4 molecules in this reaction?
c. How many moles of MnO4 - ions reacted with the given amount of oxalic acid solution?
d. How many moles of H2C2O4 were present?
e. What was the molarity of the oxalic acid solution?
f. If the density of the oxalic acid solution was 1.00 g/mL, what was the percentage by mass of oxalic acid in the solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2
You know the right answer?
A sample of oxalic acid is titrated with a standardized solution of KMNO4. A 25 mL sample of oxalic...
Questions

Mathematics, 11.10.2020 15:01

Mathematics, 11.10.2020 15:01

Mathematics, 11.10.2020 15:01

Mathematics, 11.10.2020 15:01

Mathematics, 11.10.2020 15:01


History, 11.10.2020 15:01


Mathematics, 11.10.2020 15:01



Business, 11.10.2020 15:01

Social Studies, 11.10.2020 15:01


Mathematics, 11.10.2020 15:01



Mathematics, 11.10.2020 15:01


Physics, 11.10.2020 15:01

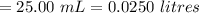
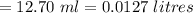
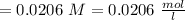
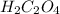 molecules mole ratio to
molecules mole ratio to 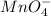 ions:
ions: 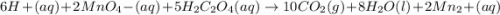
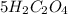 : :
: : 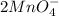
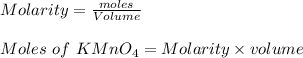
 mole ratio
mole ratio 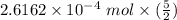
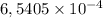 mol are present in the sample
mol are present in the sample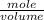
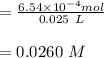
 .
.