
Chemistry, 03.01.2021 04:50 bossboybaker
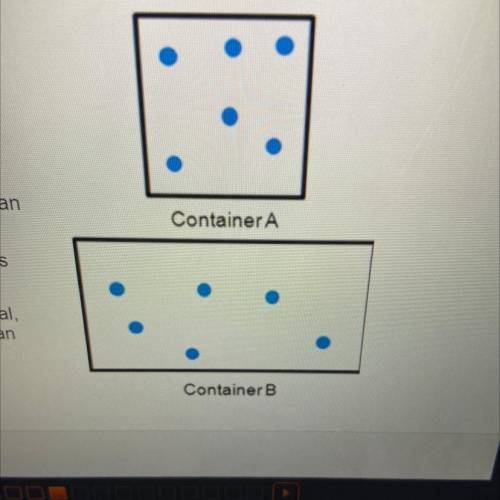
The diagrams to the right show the distribution
and arrangement of gas particles in two different
containers. According to kinetic-molecular theory,
which of the following statements is true? Check
all that apply.
If the temperatures of both containers are
equal, container A has greater pressure than
container B.
ContainerA
U
If the volume of container A decreased, its
pressure would decrease.
If the pressure in both containers is equal,
container A has a lower temperature than
container B.

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3

Chemistry, 24.06.2019 00:30
When a solute is added to a pure liquid, the boiling point increases, but the freezing point decreases. explain.
Answers: 1
You know the right answer?
The diagrams to the right show the distribution
and arrangement of gas particles in two different
Questions




Arts, 16.10.2019 21:30

Mathematics, 16.10.2019 21:30

Mathematics, 16.10.2019 21:30


Mathematics, 16.10.2019 21:30

Arts, 16.10.2019 21:30

Mathematics, 16.10.2019 21:30




History, 16.10.2019 21:30

Mathematics, 16.10.2019 21:30

Mathematics, 16.10.2019 21:30



Mathematics, 16.10.2019 21:30



