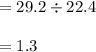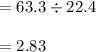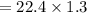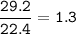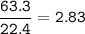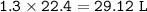Methane gas combusts according to the following
chemical equation:
CH4 (8) + 202(g) → CO2(g) + 2H2O(l)
29.2 L of methane gas is combusted with 63.3 L of
oxygen gas at STP. What volume of carbon dioxide
is produced in the reaction?
___L CO2
Your answer should be rounded to three significant figures. Do
not include units in your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Methane gas combusts according to the following
chemical equation:
CH4 (8) + 202(g) → CO2(g)...
CH4 (8) + 202(g) → CO2(g)...
Questions

Chemistry, 05.02.2020 05:53


English, 05.02.2020 05:53

Mathematics, 05.02.2020 05:53



Mathematics, 05.02.2020 05:53

English, 05.02.2020 05:53


Mathematics, 05.02.2020 05:53



Spanish, 05.02.2020 05:53


Social Studies, 05.02.2020 05:53