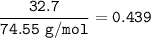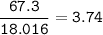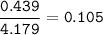An aqueous solution contains
32.7% KCI (weight/weight %).
What is the mole fraction of KCI in...

Chemistry, 31.12.2020 23:40 Chandler1Gaming
An aqueous solution contains
32.7% KCI (weight/weight %).
What is the mole fraction of KCI in
this aqueous solution?
Molar Mass
KCI: 74.55 g/mol
H2O: 18.016 g/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Questions

Mathematics, 23.01.2020 04:31


Mathematics, 23.01.2020 04:31

Health, 23.01.2020 04:31

Mathematics, 23.01.2020 04:31


Mathematics, 23.01.2020 04:31




English, 23.01.2020 04:31

Mathematics, 23.01.2020 04:31



Advanced Placement (AP), 23.01.2020 04:31


History, 23.01.2020 04:31