
Chemistry, 30.12.2020 09:00 gallowayryan19
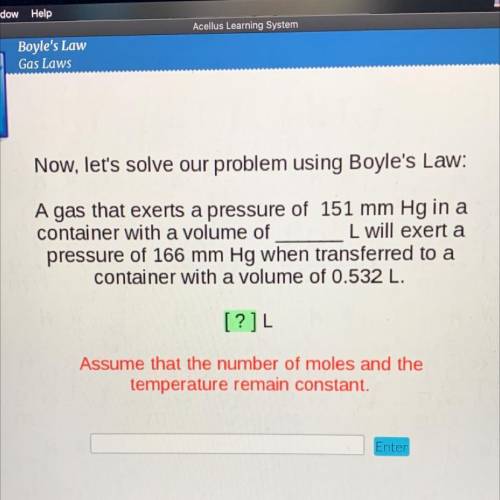
Now, let's solve our problem using Boyle's Law:
A gas that exerts a pressure of 151 mm Hg in a
container with a volume of
L will exert a
pressure of 166 mm Hg when transferred to a
container with a volume of 0.532 L.
[?]L
Assume that the number of moles and the
temperature remain constant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
Now, let's solve our problem using Boyle's Law:
A gas that exerts a pressure of 151 mm Hg in a
Questions

History, 01.12.2020 19:40

Mathematics, 01.12.2020 19:40


Mathematics, 01.12.2020 19:40

Mathematics, 01.12.2020 19:40

Arts, 01.12.2020 19:40

History, 01.12.2020 19:40




Chemistry, 01.12.2020 19:40

Social Studies, 01.12.2020 19:40

History, 01.12.2020 19:40

Biology, 01.12.2020 19:40


Business, 01.12.2020 19:40


Mathematics, 01.12.2020 19:40

Mathematics, 01.12.2020 19:40

English, 01.12.2020 19:40



