
Chemistry, 29.12.2020 22:00 jessicaaaamartin
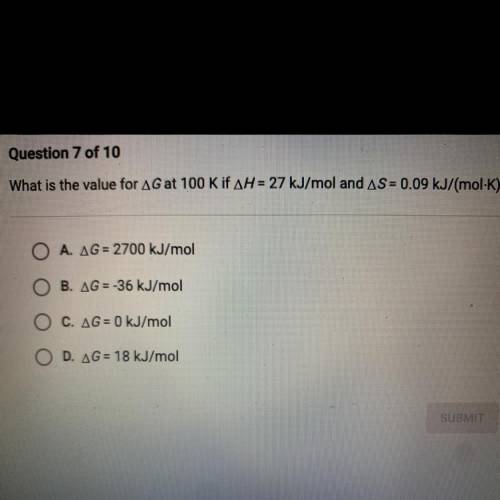
What is the value for AG at 100 K if AH = 27 kJ/mol and AS = 0.09 kJ/(mol-K)?
A. AG= 2700 kJ/mol
B. AG = -36 kJ/mol
C. AG = 0 kJ/mol
D. AG = 18 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1

You know the right answer?
What is the value for AG at 100 K if AH = 27 kJ/mol and AS = 0.09 kJ/(mol-K)?
A. AG= 2700 kJ/mol
Questions



English, 28.03.2020 03:18

Social Studies, 28.03.2020 03:18










English, 28.03.2020 03:18

Mathematics, 28.03.2020 03:18

Mathematics, 28.03.2020 03:18

Biology, 28.03.2020 03:18

Geography, 28.03.2020 03:18


Mathematics, 28.03.2020 03:18



