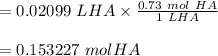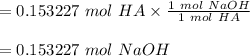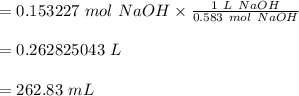Chemistry, 29.12.2020 01:00 maksimgelmud7
You have a vinegar solution you believe to be 0.73 M. You are going to titrate 20.99 mL of it with a NaOH solution that you know to be 0.583 M. At what volume of added NaOH solution would you expect to see an end point

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
You have a vinegar solution you believe to be 0.73 M. You are going to titrate 20.99 mL of it with a...
Questions

English, 29.08.2021 01:00

Mathematics, 29.08.2021 01:00



English, 29.08.2021 01:00

Mathematics, 29.08.2021 01:00


Mathematics, 29.08.2021 01:00


English, 29.08.2021 01:00



English, 29.08.2021 01:00



Mathematics, 29.08.2021 01:00


Mathematics, 29.08.2021 01:00

History, 29.08.2021 01:00