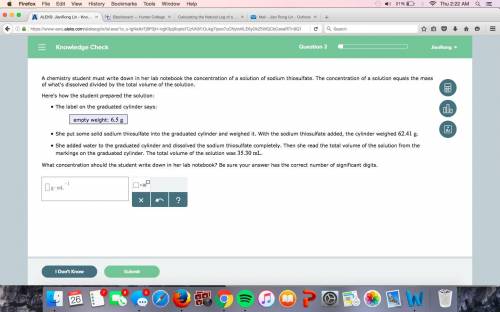
A chemistry student must write down in her lab notebook the concentration of a solution of sodium thiosulfate. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: The label on the graduated cylinder says: empty weight: She put some solid sodium thiosulfate into the graduated cylinder and weighed it. With the sodium thiosulfate added, the cylinder weighed . She added water to the graduated cylinder and dissolved the sodium thiosulfate completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was . What concentration should the student write down in her lab notebook

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

You know the right answer?
A chemistry student must write down in her lab notebook the concentration of a solution of sodium th...
Questions





Geography, 03.09.2019 05:30







Computers and Technology, 03.09.2019 05:30

Computers and Technology, 03.09.2019 05:30


Physics, 03.09.2019 05:30